
Advanced Peptide Receptor Radionuclide Therapy

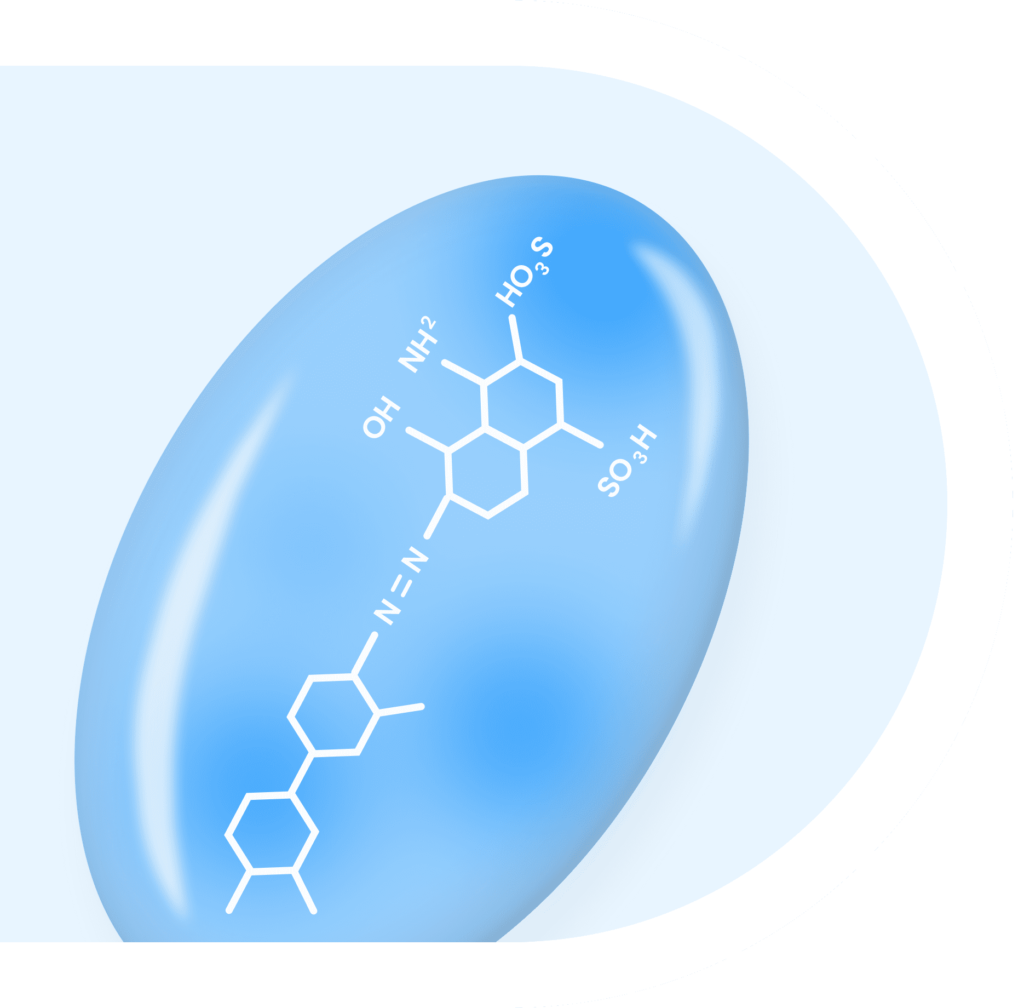
EBTATE
(177Lu-DOTA-EB-TATE and 225Ac-DOTA-EB-TATE)
EBTATE is a long-acting somatostatin analogue based on the EvaThera theranostic platform.
EBTATE uses Evans blue dye to improve pharmacokinetics and therapeutic efficacy versus established therapies. Studies to date have shown that in the treatment of gastroenteropancreatic neuroendocrine tumors (NETs), Hürthle Cell Thyroid cancer, Small Cell Lung cancer and Nasopharyngeal Cancer both tumor uptake and therapeutic effect of EBTATE was significantly greater than comparative therapies, as measured in rigorous biodistribution and SPECT imaging studies.
Early studies have established that EBTATE is safe and potentially fast-acting, with objective responses achieved after a single injection. Patients demonstrated positive tolerance to multiple cycles of escalating doses of EBTATE (up to 3.97 GBq/cycle). Additional research for the treatment of Hürtle Cell Thyroid Carcinoma (HTC) is currently underway in animal models.
3-Year Study Follow Up
In a 3-year follow-up to MTTI’s 177 Lu-EBTATE research against gastroenteropathic-neuroendocrine tumors, EvaThera has found a favorable survival outcome disease control rate of 86% and encouraging median 36-month Progression Free survival.
Comparative Findings
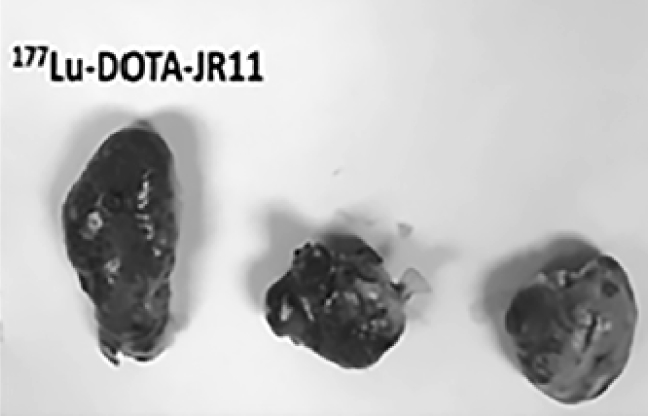
EBTATE (177Lu-DOTA-EB-TATE) is a superior therapy for treatment of Hürtle Cell Thyroid Carcinoma (HTC). In mouse models with HTC, EBTATE (left panel seen below) significantly reduced the size of SSTR2-expressing tumors and extended overall survival compared to other therapies.*
*177Lu-DOTA-EB-TATE, a Radiolabeled Analogue of Somatostatin Receptor Type 2, for the Imaging and Treatment of Thyroid Cancer, 10.1158/1078-0432.CCR-20-3453

EBTATE Benefits


Improved Uptake, Slower Clearance
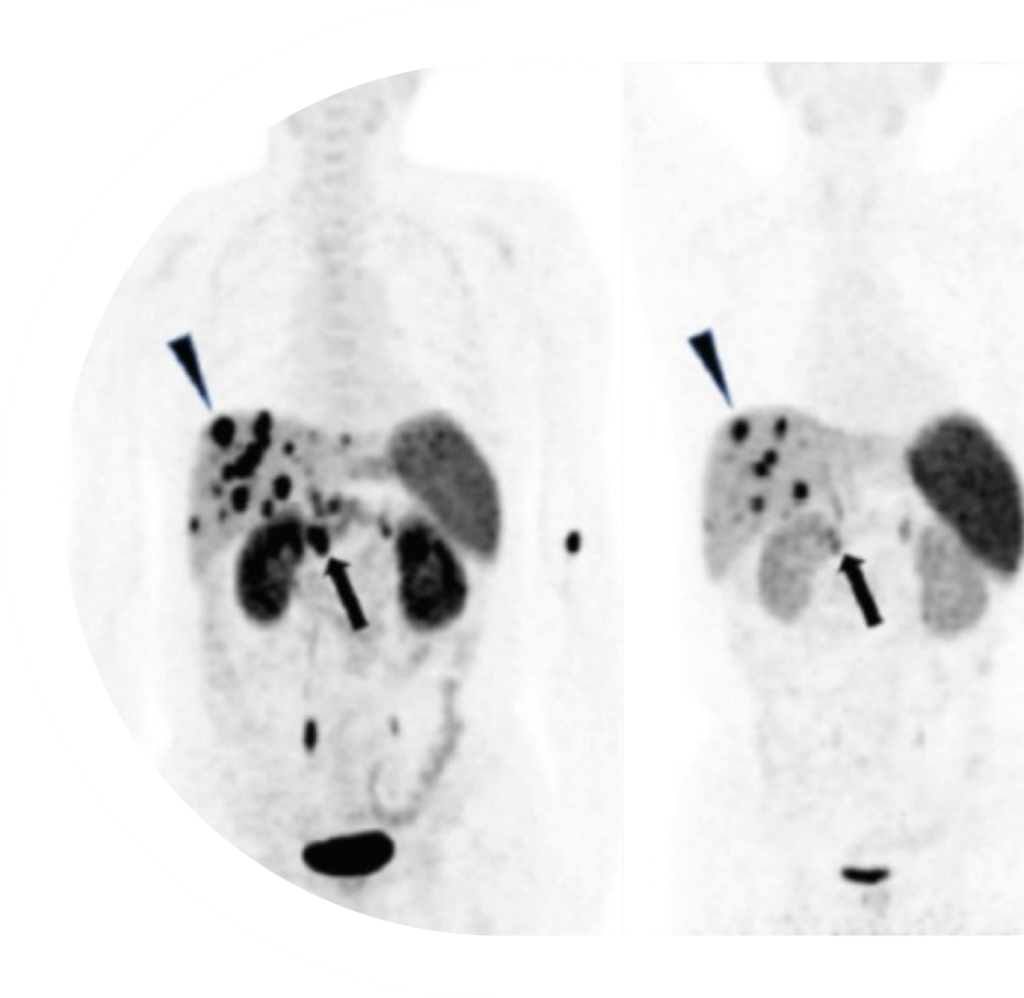
EBTATE reached peak saturation slower and had a longer plateau vs. the competing therapy.

Rapid Onset of Action
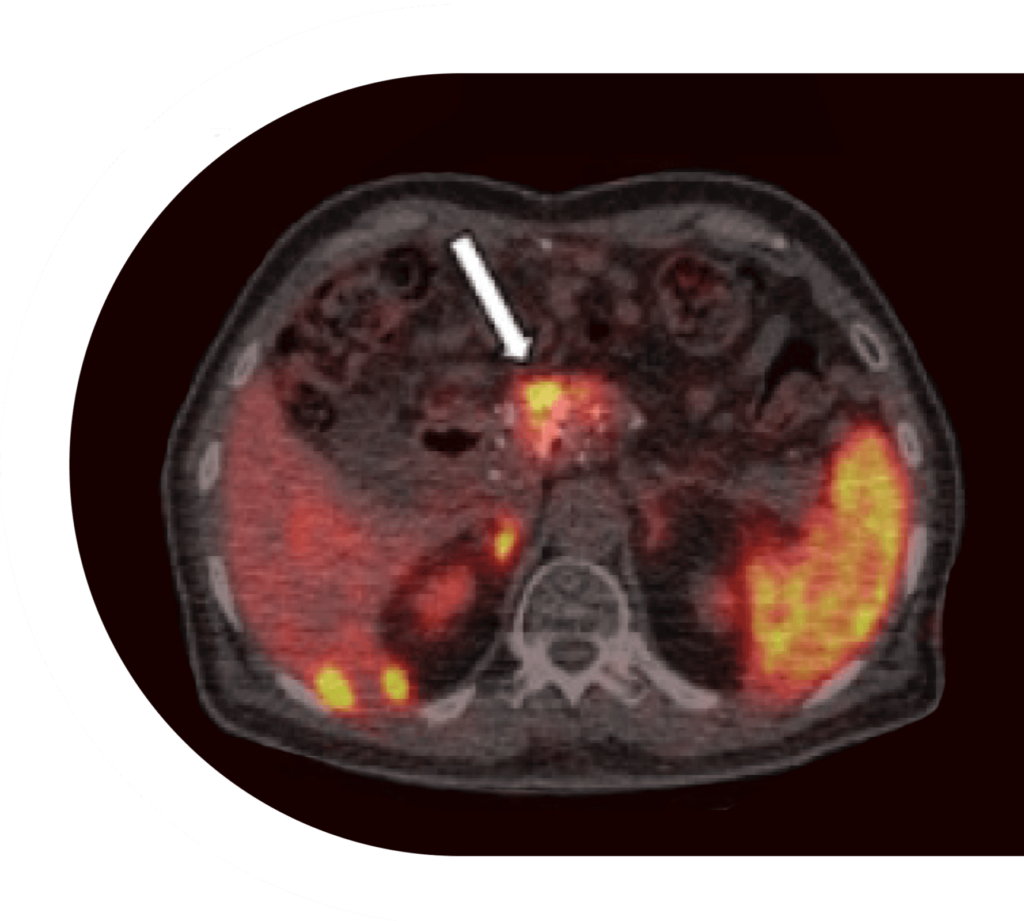
A single low dose (19.5 mCi) of EBTATE led to reductions in pancreatic NET SUVmax and highest-uptake liver metastases.

Improved Therapeutic Outcomes
EBTATE showed an 800% increase in lesion radiation vs. the competing therapy.
Be the First to Know
Visit us on LinkedIn and Connect with Us.
Data & Literature
Treatment with a single low-dose of EBTATE resulted in a striking decrease in pancreas and liver tumor size in only 3 months. Learn more about the increased efficacy of treatment in NETs using EBTATE.
Response to Single Low-dose 177Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study.

Learn more about MTTI’s innovative theranostic technology
If you have questions, or a passion for advancing radiotherapeutics and radiodiagnostics, we encourage you to consult additional resources or reach out to our team directly.

© 2023 Molecular Targeting Technologies, INC. All Rights Reserved.
PHONE
610-738-7938
EMAIL
INFO@EVATHERA.COM
