SNMMI 2022 Accepted Oral Abstract: EBTATE safety with and without Amino Acid Infusion
Evaluation of Safety, Biodistribution and Dosimetry of a long-Acting Radiolabeled Somatostatin Analogue 177Lu-DOTA-EB-TATE with and without Amino Acid Infusion
Synopsis:
Evaluation of Safety, Biodistribution and Dosimetry of a long-Acting Radiolabeled Somatostatin Analogue 177Lu-DOTA-EB-TATE with and without Amino Acid Infusion.
Qingxing Liu, Peking Union Medical College Hospital (Primary Presenter); Yuanyuan Jiang, Peking Union Medical College Hospital Koon Pak, Molecular Targeting Tech. Inc
Guochang Wang, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical Colleg Jingjing Zhang; Xiaoyuan Chen, NUS Zhaohui Zhu, Peking Union Medical College Hospital.
Purpose/Background:
Kidneys are considered one of the dose-limiting organs in peptide receptor radionuclide therapy (PRRT), especially when using 90Y-PRRT. Amino acid was infused to reduce renal absorbed dose by inhibiting the proximal tubular reabsorption of the radiopeptide. However, due to the lower penetration range of β- particles emitted by 177Lu, the renal dysfunction caused by 177Lu-PRRT is rare. An Evans blue-modified 177Lu-labeled octreotate (177Lu-DOTA-EB-TATE) have an extended circulation in the blood, making little protective effect of amino acids on the kidneys. The aim of this prospective study was to evaluate the safety, biodistribution and dosimetry of 177Lu-DOTA-EB-TATE with and without amino acid infusion. .Kidneys are considered one of the dose-limiting organs in peptide receptor radionuclide therapy (PRRT), especially when using 90Y-PRRT. Amino acid was infused to reduce renal absorbed dose by inhibiting the proximal tubular reabsorption of the radiopeptide. However, due to the lower penetration range of β- particles emitted by 177Lu, the renal dysfunction caused by 177Lu-PRRT is rare. An Evans blue-modified 177Lu-labeled octreotate (177Lu-DOTA-EB-TATE) have an extended circulation in the blood, making little protective effect of amino acids on the kidneys. The aim of this prospective study was to evaluate the safety, biodistribution and dosimetry of 177Lu-DOTA-EB-TATE with and without amino acid infusion.
Methods:
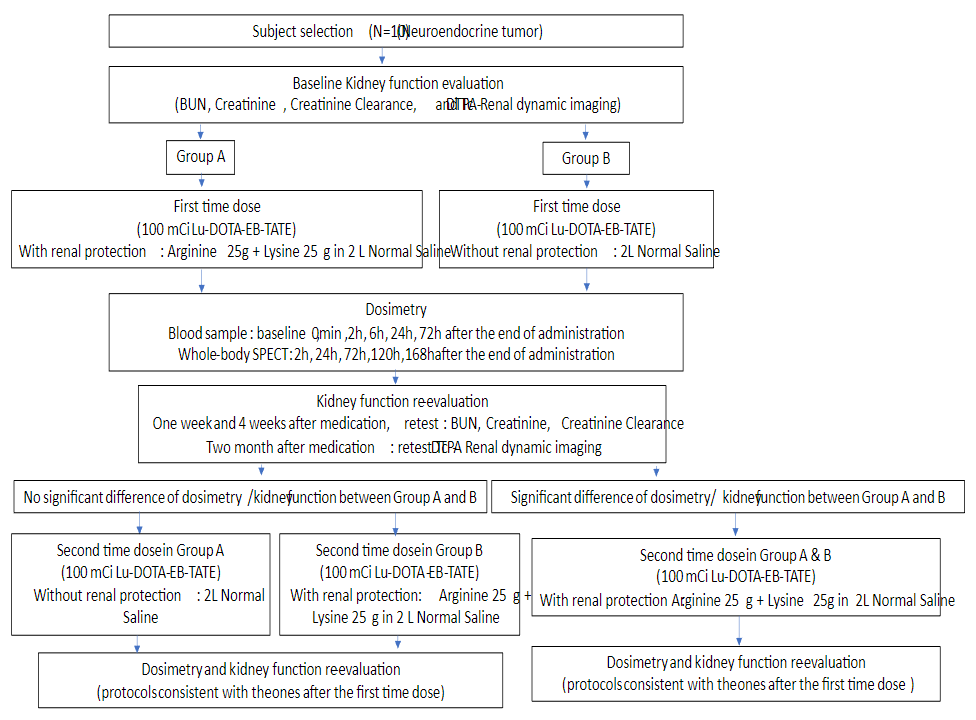
A total of 10 advanced metastatic neuroendocrine tumors were recruited and were randomly divided into 2 groups: group A (n=5, Male/Female=3/2, mean age 43±7 years old) received a single dose 3.9±0.2 GBq (105.6±4.6 mCi) of 177Lu-DOTA-EB-TATE without amino acid infusion; group B (n=5, Male/Female=3/2, mean age 56±8 years old) received a single dose 3.3±0.1 GBq (88.5±2.6 mCi) of 177Lu-DOTA-EB-TATE with amino acid infusion (25g lysine+25g arginine diluted in 2L of normal saline infused over 4 h, starting 30–60 min before PRRT). All patients underwent serial whole body planar at 1, 24, 96, 168h after administration and underwent single-photon emission computed tomography (SPECT) at 24h after administration. Abdominal CT was performed 2 days before administration for SPECT/CT image fusion. Hematological parameters, liver and renal function (creatinine and BUN) at baseline, 1 week and 4 weeks after administration were tested. 99mTc-DTPA dynamic renal imaging used for determination of the GFR was performed at baseline and 8 weeks after administration. Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Dosimetric calculations were done using the HERMES software (HERMES Medical Solutions, Stockholm, Sweden).
Results:
Administration of 177Lu-DOTA-EB-TATE with/without amino acid was well tolerated, with no CTC-3/4 hematotoxicity, hepatotoxicity or nephrotoxicity being recorded. In group A, no significant difference was observed in creatinine (t=0.485, P=0.653), BUN (t=0.585, P=0.590) or GFR (t=0.741, P=0.512). In group B, no significant difference was observed in creatinine, BUN or GFR as well. The total body effective dose has no significant difference between group A and B (0.222±0.100 vs 0.105±0.014 mSv/MBq, P=0.279) as well as kidney effective dose (2.013±0.808 vs 0.622±0.117 mSv/MBq, P=0.127). The residence time of the kidneys was 6.6±2.5h in group A and 2.1±0.4h in group B (P=0.113).
Conclusion:
177Lu-DOTA-EB-TATE without amino acid infusion is safe, with no kidney dysfunction being occurred. It could not only greatly simplify the PRRT procedure, but also avoid amino acid-related side effects.